Total video converter keygen. Jul 27, 2016 Total Video Converter 4.8 Serial Key incl There are lots of paid and completely free. software downloads that can be found on the Internet for converting AVI files in their MP4 versions. It is really a perfect choice not just for converting FLV to MP4, but additionally for converting between an extended record of SD and HD video formats. Aug 02, 2016 Free Download = Total video converter 3.71 Crack + Serial Number + Patch + Keygen. Aug 22, 2018 Total Video Converter Keygen Introduction: Total Video Converter 3.71 Key is a well-developed and well-known video converter. It has the best features. Nowadays our LCD or laptops does not support a certain video format.
| The Arbitration Committee has authorized uninvolved administrators to impose discretionary sanctions on users who edit pages related to complementary and alternative medicine, including this article. Provided the awareness criteria are met, discretionary sanctions may be used against editors who repeatedly or seriously fail to adhere to the purpose of Wikipedia, any expected standards of behaviour, or any normal editorial process. |
| This article is of interest to the following WikiProjects: | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
[Untitled][edit]
Perform a serial dilution, which are a series of dilutions, when the final volume is a large value like 10,000 mL, for example. In this case, make a 1 mL to 100 mL dilution first and from that solution take another 1 mL into another 100 mL. The final solution is a 1 to 10,000 mL (100 mL x 100 mL) dilution.
New article, briefly explaining the process. Adam Cuerdentalk 08:30, 1 February 2008 (UTC)
Cut paragraph[edit]
Homeopaths claim that such diluted solutions can have therapeutic effects despite the absence of solute. A proposed explanation invokes water memory, a controversial idea that shaking (or 'succussing') at each stage of the dilution can leave an 'imprint' of the original molecules on the solvent. However, no mechanism by which this could take place is apparent, and liquid water has been shown to lose memory of any structure within 50 femtoseconds.[1] Consequently, the proposed mechanisms of homeopathy are generally considered highly implausible by mainstream science.[2][3][4][5] Canon c5030 printer driver.
While this may be useful later, I agree that, as the article stands, this is too much information. Adam Cuerdentalk 14:30, 1 February 2008 (UTC)
- Serial dilution is also a cheaper and simpler method for preparing cultures from a single cell than optical tweezers and micro manipulators. Serial dilution is one of the core foundational practices of homeopathy, with 'succussion', or shaking, occurring between each dilution.
- . The importance of serial dilution and colony counting is reflected by the. Are often used in statistical analysis to describe the dilution process (Hedges, 2002. MPN is often used to measure microbes in milk, water and food (Blodgett, 2010 ). A serial dilution is the stepwise dilution of a substance in solution.
- Serial dilution is one of the core foundational practices of homeopathy, with 'succussion', or shaking, occurring between each dilution.In homeopathy, serial dilutions (called potentisation) are often taken so far that by the time the last dilution is completed, no molecules of the original substance are likely to remain.
- Serial dilutions are made by making the same dilution step over and over, using the previous dilution as the input to the next dilution in each step. Since the dilution-fold is the same in each step, the dilutions are a geometric series (constant ratio between any adjacent dilutions).
- Also, this article is misleading about homeopathy. After all, there is such a thing as a 1M solution using homeopathy notation, which is dilution by a factor of 100 1000 times. And then there is 1 molar solution, which is a 1 mole/ liter solution, sometimes written as 1 M as is done in this article. So this entire section is misleading confusing and a mess. See my current rough draft in the sandbox: [1]. So I have a few misgivings about this article. It is so tiny and this homeopathy section is such a scrap of forked material, that I wonder about it. Even the nonhomeopathy section needs much much more material and cites and links etc. --Filll (talk) 16:51, 1 February 2008 (UTC)
- Well, the details of which factor the serial dilution is doesn't matter much for this discussion, and we do say that it's only one example. Point of this article is to explain the basic concept of serial dilution, not to get pedantic over the eight types used in homeopathy. Adam Cuerdentalk 17:17, 1 February 2008 (UTC)
Ok fair enough. As long as one tries to avoid confusion on notation.--Filll (talk) 17:25, 1 February 2008 (UTC)
- I've changed the use of M (molar) to use mol/L instead, and added a ref. for the use of serial dilution in bacteria counting. Your draft on homeopathic concentration looks great, but I'd say that that is a different topic, which can certainly be linked from this article. This article can be about the method in general, which is used in mainstream science in many fields such as analytical chemistry, microbiology, and clinical chemistry. The article is currently short and lacks some references, but it is very new and can improve. Not all articles are born ready to be Featured Articles! --Itub (talk) 17:43, 1 February 2008 (UTC)
References
- ^Cowan ML, Bruner BD, Huse N; et al. (2005). 'Ultrafast memory loss and energy redistribution in the hydrogen bond network of liquid H2O'. Nature. 434 (7030): 199–202. doi:10.1038/nature03383. PMID15758995.Explicit use of et al. in:
|author=(help)CS1 maint: multiple names: authors list (link) - ^Anonymous (1988). 'When to believe the unbelievable'. Nature. 333 (6176): 787. doi:10.1038/333787a0.
- ^Shang A, Huwiler-Müntener K, Nartey L; et al. (2005). 'Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy'. Lancet. 366 (9487): 726–732. doi:10.1016/S0140-6736(05)67177-2. PMID16125589.Explicit use of et al. in:
|author=(help)CS1 maint: multiple names: authors list (link) - ^Ernst E, Pittler MH (1998). 'Efficacy of homeopathic arnica: a systematic review of placebo-controlled clinical trials'. Archives of surgery (Chicago, Ill. : 1960). 133 (11): 1187–90. PMID9820349.
- ^Cite error: The named reference
Ernst2005was invoked but never defined (see the help page).
Reverted[edit]
While it is mathematically correct that no molecules of original substance are present in 12C or higher potencies, only a few test tubes of water are actually used in dilution and succussion. Homeopaths believe that the repeated agitation of the water with each successive dilution causes some physical alteration of the structure of the water which they consider biologically active.
If editors are going to present a mathematical argument about relative volumes of water it should be stated that we're not actually talking about very much water at all here. —Whig (talk) 04:52, 2 February 2008 (UTC)
- This is the article about serial dilution. One suspects they'll have picked that up. Adam Cuerdentalk 05:05, 2 February 2008 (UTC)
- I don't think you can leave it implied, otherwise why include the mathematical argument at all? This seems to be presenting one side of an argument only, which goes against NPOV. —Whig (talk) 05:08, 2 February 2008 (UTC)
- Oh, please don't start that 'I'm the only arbiter of NPOV' thing again. Adam Cuerdentalk 05:10, 2 February 2008 (UTC)
- I don't think you can leave it implied, otherwise why include the mathematical argument at all? This seems to be presenting one side of an argument only, which goes against NPOV. —Whig (talk) 05:08, 2 February 2008 (UTC)
I think it is clear from
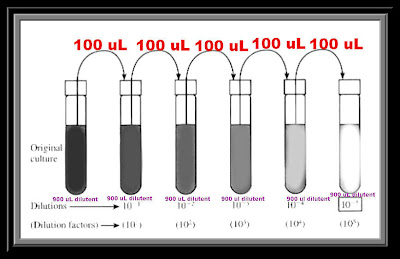
A typical serial dilution might involve a factor of ten dilution at each stage, done as follows:Start with the original substance to be diluted, at whatever starting concentration you may have it at, or which is convenient to create. 1 mol/L for example. Take 1 millilitre of it, place in a 10 millilitre volumetric flask, and top the volumetric flask. As there is now 1 mL of the original substance in 10 mL of solution, the concentration is 1/10th what it was originally, or 0.1 mol/L. Take 1 mL of the 0.1 mol/L solution, add it to another 10 mL flask, and fill that up to 10 mL. We now have 1/10th the concentration of the 0.1 mol/L solution, giving us a 0.01 mol/L solution. This process may be continued as far as the experiment or purpose requires, and (in this case) gives 9 mL of all the intermediate concentrations (10 mL less the 1 mL used to make the next dilution), which may be useful if it is desired to test at these concentrations as well.
that the volumes handled at each time are comparatively small. --Rifleman 82 (talk) 05:19, 2 February 2008 (UTC)
Is this a chemistry article?[edit]
Within chemistry M = molarity = mol/L. This is an article about a simple chemical process so 1 M should suffice to mean 1 mol/L. But it seems that some religion defines M otherwise, it can't be used in describing a chemistry process? Severe undue weight problems here.
I removed a beliefqualification from the homeopathy section - the section should be cut to no more than a one sentence see also .. for use in .. or whatever - where the beliefs and nonchemical use of M can be discussed at length - per WP:UNDUE. Vsmith (talk) 05:13, 2 February 2008 (UTC)
- I think the purpose of the procedure is important for context, but I would certainly state that this is not a chemistry issue at all nor does it claim to be. —Whig (talk) 05:15, 2 February 2008 (UTC)
- Say what? Chemical dilution is not a chemistry issue? Your logic escapes me. A simple chemicla procedure is most definitely a chemistry issue. The article describes that chemistry lab procedure quite clearly. Vsmith (talk) 05:26, 2 February 2008 (UTC)
- Homeopathic potentization is not a chemistry procedure. —Whig (talk) 05:27, 2 February 2008 (UTC)
- This is not an article to discuss homeopathic potentization. It is merely describing a simple procedure common in the physical sciences, *especially chemistry*, to dilute substances. The identity of the substance is really, irrelevant. Discussion on homeopathic use, beyond a brief mention, should go to Homeopathy#Dilution_and_succussion. --Rifleman 82 (talk) 05:44, 2 February 2008 (UTC)
- As it stands we are presenting a mathematical argument against homeopathy. This goes against NPOV if we present only one side of such an argument, and I agree the whole argument is unnecessary and should be removed but when I did so I was reverted. [2] —Whig (talk) 05:54, 2 February 2008 (UTC)
I thought the whole point of homeopathy was that it does not use serial dilution, but serial dilution AND succussion? I'd favor a minor mention in See also. David D.(Talk) 06:07, 2 February 2008 (UTC)
- I'd agree with that. —Whig (talk) 06:10, 2 February 2008 (UTC)
- Well, it is half of the key procedure of homeopathy. A brief discussion seems necessary. Adam Cuerdentalk 06:18, 2 February 2008 (UTC)
- Looks more like you're trying to make a point. David D.(Talk) 06:35, 2 February 2008 (UTC)
- It was mentioned that it possibly should be included on WT:WikiProject Chemistry. So I tried to work it in. Adam Cuerdentalk 06:50, 2 February 2008 (UTC)
- Yet, as a biologist yourself, you get homeopathy in there right away but there is no mention of phage plaques or bacteria counting until a later version? Interesting. David D.(Talk) 06:53, 2 February 2008 (UTC)
- I added a section on it in biology soon after. I was inpspired to write the homeopathy by Itub's comment. dmittedly, Itub said that all the troubles on homeopathy might be a reason to avoid it, but I foolishly thought that the probation would calm such edit wars and other such things down sufficiently that we could talk about a genuinely relevant topic, without ending up with a hostile environment. Adam Cuerdentalk 06:57, 2 February 2008 (UTC)
- You were inspired? A genuinely relevant topic? Has this leopard changed his spots? David D.(Talk) 07:02, 2 February 2008 (UTC)
- The above comment is uncivil and certainly is not in the spirit of WP:AGF. It may well be actionable under the terms of the homeopathy article probation. Please help calm things down, instead of worsening the situation through personal attacks. Raymond Arritt (talk) 09:56, 2 February 2008 (UTC)
- There's a huge difference between a core concept of homeopathy and a mere 'it is used by some homeopaths'. ALL homeopathic remedies use serial dilution. Not all use Potassium dichromate. Adam Cuerdentalk 07:08, 2 February 2008 (UTC)
- And why do you think a paragraph on homeopathy here will have the distinction of 'without ending up with a hostile environment' given it was written as an exposé? David D.(Talk) 07:12, 2 February 2008 (UTC)
- You were inspired? A genuinely relevant topic? Has this leopard changed his spots? David D.(Talk) 07:02, 2 February 2008 (UTC)
- I added a section on it in biology soon after. I was inpspired to write the homeopathy by Itub's comment. dmittedly, Itub said that all the troubles on homeopathy might be a reason to avoid it, but I foolishly thought that the probation would calm such edit wars and other such things down sufficiently that we could talk about a genuinely relevant topic, without ending up with a hostile environment. Adam Cuerdentalk 06:57, 2 February 2008 (UTC)
- Yet, as a biologist yourself, you get homeopathy in there right away but there is no mention of phage plaques or bacteria counting until a later version? Interesting. David D.(Talk) 06:53, 2 February 2008 (UTC)
- It was mentioned that it possibly should be included on WT:WikiProject Chemistry. So I tried to work it in. Adam Cuerdentalk 06:50, 2 February 2008 (UTC)
- Looks more like you're trying to make a point. David D.(Talk) 06:35, 2 February 2008 (UTC)
- Well, it is half of the key procedure of homeopathy. A brief discussion seems necessary. Adam Cuerdentalk 06:18, 2 February 2008 (UTC)
(unindent) David, you're verging on bad faith here. If there's a problem with the article, focus on the article, not on other editors. Whatever may have been, this article is best benefited by working on the article. FT2(Talk | email) 12:53, 2 February 2008 (UTC)
Edit[edit]
I've removed the second paragraph, which seemed to be unhelpful. Basically, this is an article on a method of dilution, not a staging post for the dispute on homeopathy. The introduction states what serial dilution means, the first paragraph of this section states it's used in homeopathy in a way that 'no molecules of the original substance are likely to remain'. The second paragraph then says the exact same again as an example, and adds nothing more, except for the impression it's trying to re-make the point. No need. It already says everything. For more information, direct readers to Homeopathy, whjich is now linked as the main article. Looks better balanced now. Hope that helps?
FT2(Talk | email) 12:44, 2 February 2008 (UTC)
- Sounds good. David D.(Talk) 13:30, 2 February 2008 (UTC)
- Sure. Adam Cuerdentalk 14:47, 2 February 2008 (UTC)
- Seems we all agree. —Whig (talk) 17:18, 2 February 2008 (UTC)
- Sure. Adam Cuerdentalk 14:47, 2 February 2008 (UTC)

A serial dilution is the stepwise dilution of a substance in solution. Usually the dilution factor at each step is constant, resulting in a geometric progression of the concentration in a logarithmic fashion. A ten-fold serial dilution could be 1 M, 0.1 M, 0.01 M, 0.001 M .. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale. A tenfold dilution for each step is called a logarithmic dilution or log-dilution, a 3.16-fold (100.5-fold) dilution is called a half-logarithmic dilution or half-log dilution, and a 1.78-fold (100.25-fold) dilution is called a quarter-logarithmic dilution or quarter-log dilution. Serial dilutions are widely used in experimental sciences, including biochemistry, pharmacology, microbiology, and physics.
In biology and medicine[edit]
In biology and medicine, besides the more conventional uses described above, serial dilution may also be used to reduce the concentration of microscopic organisms or cells in a sample. As, for instance, the number and size of bacterial colonies that grow on an agar plate in a given time is concentration-dependent, and since many other diagnostic techniques involve physically counting the number of micro-organisms or cells on specials printed with grids (for comparing concentrations of two organisms or cell types in the sample) or wells of a given volume (for absolute concentrations), dilution can be useful for getting more manageable results.[1] Serial dilution is also a cheaper and simpler method for preparing cultures from a single cell than optical tweezers and micromanipulators.[2]
In homeopathy[edit]
Serial dilution is one of the core foundational practices of homeopathy, with 'succussion', or shaking, occurring between each dilution. In homeopathy, serial dilutions (called potentisation) are often taken so far that by the time the last dilution is completed, no molecules of the original substance are likely to remain.[3][4]
See also[edit]
Importance Of Serial Dilution Calculator
References[edit]
- ^K. R. Aneja. Experiments in Microbiology, Plant Pathology and Biotechnology. New Age Publishers, 2005, p. 69. ISBN81-224-1494-X
- ^Booth, C.; et al. (2006). Extremophiles. Methods in microbiology 35. Academic Press. p. 543. ISBN978-0-12-521536-7.
- ^Weissmann, Gerald (2006). 'Homeopathy: Holmes, Hogwarts, and the Prince of Wales'. The FASEB Journal. 20 (11): 1755–1758. doi:10.1096/fj.06-0901ufm. PMID16940145. Retrieved 2008-02-01.
- ^Ernst, Edzard (November 2005). 'Is homeopathy a clinically valuable approach?'. Trends in Pharmacological Sciences. 26 (11): 547–548. CiteSeerX10.1.1.385.5505. doi:10.1016/j.tips.2005.09.003. PMID16165225.

- Michael L. Bishop, Edward P. Fody, Larry E. Schoeff. Clinical Chemistry: Principles, Procedures, Correlations. Lippincott Williams & Wilkins, 2004, p. 24. ISBN0-7817-4611-6.
Importance Of Serial Dilution Test
External links[edit]
- How to Make Simple Solutions and Dilutions, Bates College